Paradigm Global Events is very pleased to welcome you to our upcoming Patient Centricity and Collaboration World Congress 2024 Americas.
The two-day Congress aims to build meaningful collaborations within the industry, advocacy groups, clinicians, researchers, and most importantly, patients and their caregivers. Gain practical strategies and best practices on challenges, innovations, technologies, and concepts in achieving this goal.
The congress focuses on “Patient empowerment and understanding to inspire and create a meaningful impact”.
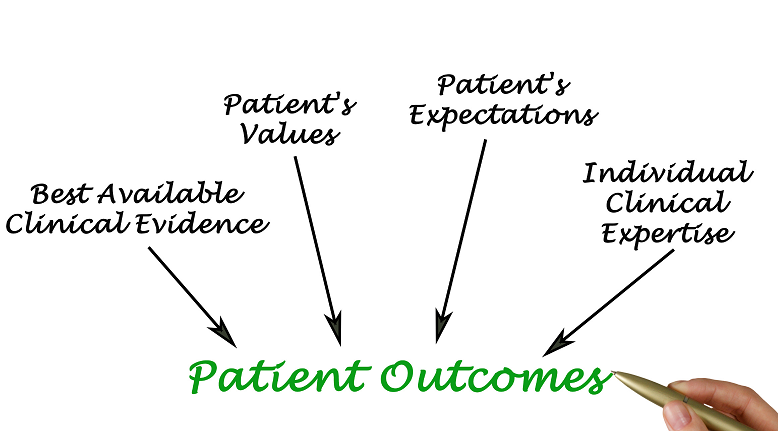
Patient-centricity is a vital aspect in the research and development of biopharmaceutical products, disease management, designing a treatment, clinical trial, or other health solutions. In order to create a patient-centric solution, one must truly embrace a collaborative endeavor with the patient and their caregivers. Establishing a patient-centric solution involves getting feedback from real patients and their loved ones, and making decisions based on their medical conditions, experiences, needs, perspectives, and priorities.
In the current climate where the patients are increasingly becoming more empowered, life sciences and pharmaceutical companies should confront the status quo and welcome the opportunities to embrace a wide range of patient-centered perspectives presented by an emerging ecosystem.
While the technology-driven world poses challenges, it also holds opportunities to build a positive patient experience by coordinating the health ecosystem to centre on the patient. Reevaluating how it develops and delivers drugs to improve experiences and outcomes and also to continuously adapt and transform to meet the evolving needs of patients.
The industry has come a long way with utilizing a patient-centered approach. The concept has gone from a buzzword to a potential trend, to a perceivable goal.
Whilst the industry has advanced in recent years, the evolving healthcare model and ecosystem brought forward by advances in technology and more involved, empowered patients, means true patient centricity is a continuing quest and a compelling commitment.
We look forward to meeting you at the Congress!
Sincerely yours,
Jocelyn Raguindin
Conference Director
Paradigm Global Events
GAIN LATEST INSIGHTS ON:
- What does patient centricity mean for the pharmaceutical and life science industry?
- Why does the industry need to be a part of the patient-centric movement?
- Patient-centric initiatives that have a tremendous impact
- Successful strategies and best practices to successfully manage patient-centricity in healthcare
- How to develop and execute a strategy to improve patient engagement?
- Benefits of patient engagement in drug development and discovery process
- Evolving challenges and key considerations in the ethical and compliant use of relevant data
- The potential value of providing access to relevant data to all stakeholders
- How to maintain mutually beneficial ongoing partnerships between the patients and industry?
- Overcoming myriad challenges to make the clinical trial and drug development process more patient-centred or patient-focused
- Setting the pace and expectations thru collaborations
- Role of researchers in bridging the communication gap between life science industry and the patient it serves
- How to achieve a deeper understanding of the patient experience in the real world.
- Practical ways in putting the patient at the centre of a powerful brand
WHO SHOULD ATTEND?
This Congress is beneficial to patients, pharmaceutical, biotech companies, researchers, physicians, patient advocacy groups, regulatory agencies, technology and healthcare companies.
Network with Presidents, Heads/Chiefs, VPs, Directors, and Managers in the area of:
- Patient Engagement
- Patient Services
- Engagement Strategy
- R&D Patient Engagement
- Medical Affairs
- Commercialisation
- Marketing
- Regulatory Affairs and Policy
- Patient Support
- RWE, and Data Management
- Quality and Compliance
- Clinical Development
- Programme Management
- Supply Chain Management
- Patient Access
- Clinical Research
- Digital Accelerator
- Patient Engagement &
- Portfolio Strategy
- Patient Support Strategy & Insights
- Patient Experience
- Global Patient Advocacy & Alliances
- Government Policy and Advocacy
- Digital Patient
- Experience Lead
- Clinical Operations
- Clinical Insights and Experience
- Head of Strategy, Access Services
- Vice President, Site Collaborations and Patient
Centricity - Head of Neuroscience
- And much more…
- Day 1 30/10/2024
- Day 2 31/10/2024
- Auditorium 1
TRENDS & STRATEGIES IN INTEGRATING PATIENT VOICE
- The value of engaging and communicating with the patient in every part of the clinical research process
- Examining the right approach for patient and industry to positively impact healthcare costs and patient outcomes
through patient engagement - How can patient expertise create significant value to the industry?
- What are the best practices and strategies to guarantee that patient centricity is at the forefront of pharma?
Moderator:
Panelist:
- Many biotech/pharmaceutical companies are talking about ”Patient Centricity”, but how can a company actually operate
in a patient-centric way to create meaningful value? - Learn best practices to transition from being product centered to patient centered
- Discuss strategies to embed patient centricity throughout your organization.
- Patient Centricity requires new mindsets, relationships – and structures, company-wide.
- No stage of development, from discovery through delivery, gets a pass.
- No company function continues as before.
- Company culture changes, too.
- This isn’t easy but the results are worth the journey: to improve outcomes for patients and business performance alike.
- If Patient Centricity is a fad, then it’s a failure. It should be the new way of doing business.
- Sharing insights into a live real-world example of this type of partnership
- Explaining the roles and responsibilities in achieving a positive outcome
- Providing key learning to the evolving process
- Supplying the audience with guiding principles for future partnerships
- Learn about the latest technologies that can improve the efficiency and effectiveness of clinical trial design, addressing issues like increasing complexity
Discover the principles and best practices for clinical trial design that can increase the likelihood of success, through a combination of literature analysis and practical experience - Examine the challenges faced by patients during clinical trials and learn about potential solutions that can reduce the burden and increase the benefits for patients
- Understand where patient centricity can advance the interests shared by industry and the patient community
- Consider how focused goals and programs can strengthen research and development, commercialization and access
- Focus on opportunities to integrate patients, caregivers, consumers and advocates’ key concerns and insights into company plans and decision making
- Discuss applications of this targeted approach to your patient centricity goals and programs
TOPIC FOCUSED STREAM
ACCESS & COLLABORATIONS
- Health Systems sharing clinical data to advance science
- Patient Advocacy Group’s use of multi-stakeholder
registries - 21st Century Cures Act enabling patients to have access to
all their clinical data and agency to license - Combined with emerging trusted data market approaches
- Creates the opportunity for patient-centric ecosystems at
scale to accelerate discovery -> clinical adoption
- Learning: Balancing health system priorities and resources to address patient community needs.
- Creating policy to end disparities and open access to new regenerative and translational therapies.
- Increasing funding and technical support to CBOs for community outreach and education efforts.
- Financial Toxicity During Breast Cancer Treatment: A Qualitative Analysis to Inform Strategies for Mitigation
- Have patient “Advisors” on a panel for clinical trials.
- Have a patient advocate/liaison collaborating.
- Patient Surveys
- Education of the process of clinical trials
- Listen to the Patient needs and help them to understand what the focus of the trial is.
- Understanding Pharmaceutical costs of trials and research
- Dealing with an illness with multiple organ involvement.
- Rare Disease
- How we might innovate our way out of increasing erosion of trust between patients and “the system”, by learning from CPG use cases, nonprofit communications, digital health solutions, shifting trends in consumer media consumption, shared accountability, and increased federal protections.
RESEARCH & DEVELOPMENT
- Integration of the patient perspective into the development process
- Challenges and strategies for improving study protocols
- Streamline your clinical trial process to drive enrollment
- Patients and caregivers know best when it means improving the quality of life.
- Reality is much different than theory.
- Benefits of real-world experience inserted in basic science equals endpoints and outcomes that can be overlooked as unimportant.
- Across the spectrum of disease, from common to rare, patient data is becoming a critical driver of drug discovery, development, diagnosis,
commercialization and inclusive and equitable access to trials and treatments.
- Contributions that advocacy groups can make to clinical trial development
- The importance of knowing the people, their disease, and its burdens
Successful collaborations are a win for all
- Patient perception and usability of drug products is critical for efficacy of prescribed treatments.
- Drug products should be designed to meet the specific needs of the target patient populations.
- A patient centric drug product design approach is critical to support optimal therapeutic outcomes for drug products.
- Roll out of digital technology will support the implementation of a patient centric mindset for drug product design.
- Case-studies for drug products developed via patient centric drug product design
- Across the spectrum of disease, from common to rare, patient data is becoming a critical driver of drug discovery, development, diagnosis, commercialization and inclusive and equitable access to trials and treatments.
- Auditorium 1
CHALLENGES & POTENTIALS OF PATIENT ENGAGEMENT
- Why diversity and inclusion in clinical trials are crucial?
- Understanding critical barriers to minority and underserved patient communities participation in clinical trials
- Equity and Inclusion of Rare Disease Patients – A Must on Many Levels
- What can the industry do to promote more diversity in the future?
- Latest guidelines that make healthcare more inclusive
Moderator:
Panelists:
- How knowledge can reduce the fear of Pharma and ultimately save lives.
- What do we really know about patient safety, both as an industry and an individual and what are the challenges we face?
- How can technology play a role in ensuring patient safety, compliance and pharmacovigilance?
- How we can leverage individual and collective data to ensure proper care and Quality of life while reducing damage, saving time and money.
- Patient engagement activities incorporating robust and meaningful patient engagement experiences to inform
regulatory work
Considerations in patient engagement
Resources to help engage with FDA
- Support different learning styles to ensure patients have equal access to education
- Patient centricity for better health outcomes
- How to determine cultural nuances and understandings to care
- The importance of data democratization
- Common challenges in clinical data review that hinders clinical trials
- Strategies in overcoming these challenges
- How to seamlessly integrate data from a variety of sources?
- How to improve safety review efficiencies and reduce the timeline to critical studies?
- Understanding complexities and addressing challenges faced by the industry in setting up end-to end supply chain for cell and gene therapies
- Best practices and lessons learned that guarantees effective manufacturing and delivery of CGTs to the patients who needs them the most
- Robust technologies and innovative platforms that connects therapies with patients both in clinical and commercial scale
This presentation will highlight how companies can advance health equity through people- and patient- centered frameworks. Learning objectives include:
- Specific domains of action for addressing health equity through people and patient engagement;
- Success measures of efforts to advance health equity;
- Different interpretations of decision making across stakeholders
- Value on patient outcomes and engagement (case studies)
- Introduce the working model of decision making.
- Putting the patient first achieves the best experience and outcome for that person and their family
- Improve communication from staff shift-changes in a hospital to getting the patient discharged faster
- Setting expectations soon
- Updating goals and outcomes; timely
- Recognising key areas to ensure patients’ experiences, perspectives, needs, and priorities are captured and meaningfully incorporated into drug development and evaluation.
- Patient’s input to improve quality, relevance, safety and efficacy of drug development
- Challenges and opportunities for patient-centric product design
- The role of respective stakeholders and the way they interact, from the early steps of drug development to access inreal life?
Moderator:
Panelists:
- Understanding the drivers for innovation in pharma
- Partnering with patients and placing patient well-being at the core of all initiatives
- Real-world data and patient-reported outcomes presents the power and the potential to redesign healthcare
- Innovative approaches for direct-to-patient assume significant importance for driving patient-centricity
- Technologies that enhance the patient-centricity in pharma
Moderator:
Panellist:
